How Does Ion Exchange Work? Key Steps Explained
Water treatment is essential for producing clean, safe water for all industries and municipalities. One of the most effective methods to remove dissolved salts, hardness minerals, and other problematic ions is through ion exchange. But exactly how does ion exchange work, and why is it such a critical technology in water purification?
Ion exchange is a chemical process that swaps undesirable ions in water for more acceptable ones. Using specially designed materials called ion exchangers, this process helps soften water, remove contaminants, and prevent scale formation. By exchanging ions at the molecular level, it improves water quality and protects equipment from damage caused by hardness or corrosive substances.
In this article, we will break down how ion exchange works step by step, explain the key components and resins involved, and explore how proper maintenance ensures long-term efficiency. You’ll also learn how EAI applies ion exchange technology to help facilities achieve sustainable and cost-effective water treatment.
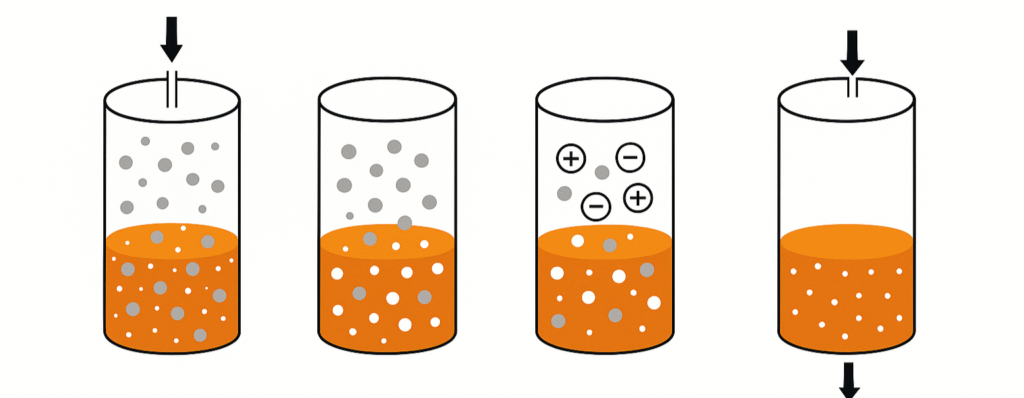
What Is Ion Exchange?
At its simplest, ion exchange is a chemical process where certain ions in water are replaced with others that are less problematic or more desirable. This exchange occurs when water flows over materials known as ion exchangers, which are engineered to attract and hold specific ions while releasing others into the water.
The Basic Exchange Process
Water contains many dissolved salts and dissolved ions, such as calcium ions, magnesium ions, chloride ions, and sodium ions. Some of these contribute to hardness, scaling, or corrosion in equipment. During ion exchange:
- The water passes through a resin bed packed with resin beads.
- The beads are covered in exchangeable ions — often hydrogen, hydroxide, or sodium.
- As water flows through, the undesirable or problematic ions in the water swap places with the ions held by the resin.
For example:
- In cation exchange, positively charged ions (cations) like calcium and magnesium are replaced by sodium or hydrogen.
- In anion exchange, negatively charged ions (anions) such as chloride or sulfate are replaced by hydroxide.
This exchange process helps deliver water that is softer, purer, and safer for use in industrial systems, water softening applications, and even drinking water production.
By combining the right ion exchangers, operators can remove a wide range of contaminants — even targeting specific desirable ions to improve water quality or meet regulatory requirements.
Key Components of Ion Exchange Systems
An effective ion exchange system relies on more than just the chemical reaction itself. Its design and components determine how well the system can treat water, sustain flow rates, and maintain efficiency over time. Below are the main parts and their roles.
The Resin Bed and Resin Beads
At the heart of any ion exchange system is the resin bed, a packed column of tiny resin beads. These beads are made from a polymer matrix (usually a cross-linked resin structure) that is durable, porous, and chemically stable.
- The resin matrix provides mechanical strength and creates a network of pathways for water and ions to travel through.
- The polymer matrix or polymer structure is engineered to resist breakdown over time while keeping pores open for ion access.
- The resin structure also determines swelling, shrinkage, and resistance to fouling.
Functional Groups
Attached to the resin beads are specific functional groups, such as sulfonic acid or quaternary ammonium, that facilitate ion exchange. These groups are responsible for attracting and holding either positively charged cations (like calcium and magnesium) or negative ions (like chloride or sulfate) during the exchange reaction.
Exchange Sites and Physical Properties
Within each bead are numerous exchange sites, where exchangeable ions reside until displaced by ions from the water.
- Properties such as physical properties, porosity, and bead uniformity directly affect how well the system operates.
- High surface area and proper bead sizing ensure maximum contact between water and resin, improving the removal of scale forming ions, hardness ions, and other problematic ions.
These carefully engineered components work together to provide reliable and efficient water treatment, whether the goal is water softening, removing dissolved ions, or producing ultra-pure water.
The Ion Exchange Process: Step-by-Step
So, how does ion exchange work in practice? This section breaks the process down into clear steps, showing how water passes through an ion exchange system and how ions are swapped efficiently.
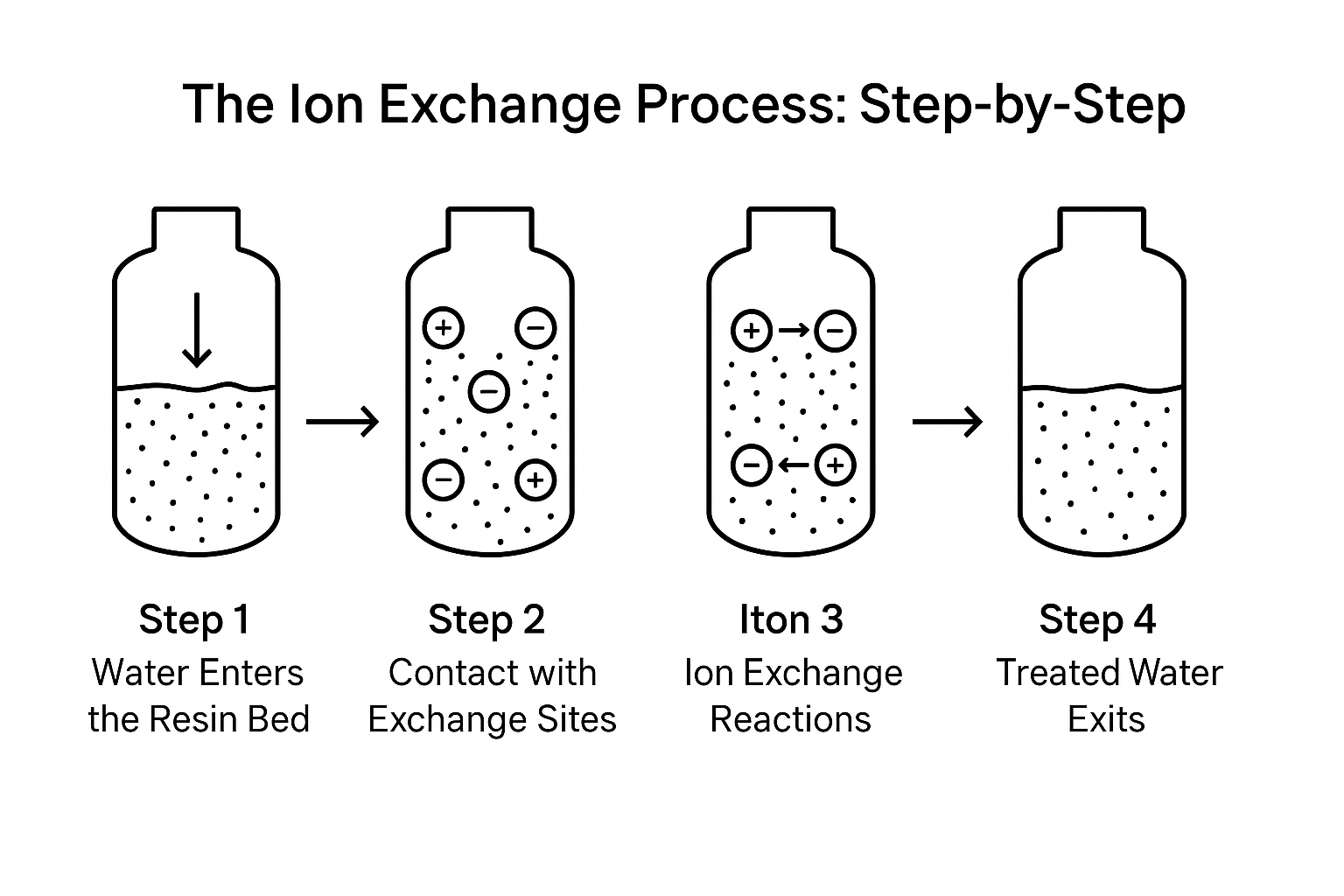
Step 1: Water Enters the Resin Bed
Raw water containing dissolved salts, hardness ions, and other impurities flows into the ion exchange column. The column contains a resin bed filled with millions of resin beads, ready to interact with the water.
Step 2: Contact with Exchange Sites
As water passes through, its dissolved ions — such as calcium and magnesium (cations) or chloride and sulfate (anions) — come into contact with the exchange sites on the resin beads. These sites are coated with exchangeable ions that are held lightly enough to be replaced by ions from the water.
Step 3: Ion Exchange Reactions
Here the chemical exchange reaction occurs:
- In cation exchange, positively charged ions like calcium, magnesium, or sodium swap places with hydrogen or sodium already on the resin.Example: Ca²⁺ + 2Na⁺-Resin → Ca²⁺-Resin + 2Na⁺
- In anion exchange, negatively charged ions like chloride or carbonic acid are replaced by hydroxide or other desirable ions.Example: Cl⁻ + OH⁻-Resin → Cl⁻-Resin + OH⁻
This facilitates ion exchange reactions continuously as water moves through the column.
Step 4: Treated Water Exits
The water, now stripped of problematic ions, leaves the system with improved quality. Remaining ions in the water are either harmless or significantly reduced, depending on the design of the ion exchange technology.
Example Applications
- Cation exchange process removes hardness ions (calcium sulfate, magnesium ions) to prevent scale.
- Anion exchange helps reduce negative ions that contribute to corrosion or fouling.
- Together, these processes produce water suitable for water softening, industrial use, or even drinking.
This step-by-step exchange process ensures the selective removal of undesired ions while retaining or adding less problematic ions or even desirable ions in the water.
Types of Resins and Their Roles
Different water treatment goals require different types of resins. Choosing the right resin ensures that the ion exchange process targets the correct ions, works efficiently, and meets the system’s water quality demands.
Cation Exchange Resins
These resins exchange positively charged cations in the water, such as calcium ions, magnesium ions, and sodium ions, for hydrogen or sodium held by the resin.
Two main types of cation resins:
- Strong acid cation resins:
- Highly effective across a broad pH range.
- Used in water softening, removing hardness ions and scale forming ions.
- Weak acid cation (WAC) resins:
- Selective for certain cations and operate better at higher pH.
- Ideal for removing alkalinity or specific metals.
Anion Exchange Resins
These resins replace negatively charged ions, such as chloride ions, sulfates, and carbonic acid, with hydroxide ions.
Two main types of anion resins:
- Strong base anion exchangers:
- Remove a broad range of anions, including weakly dissociated ones.
- Suitable for demineralization and removing dissolved ions to produce ultra-pure water.
- Weak base anion resins:
- Primarily remove strong acids and are more efficient in specific applications.
Factors That Influence Resin Selection
- Ion exchange capacity: How many ions the resin can exchange before regeneration.
- Functional groups: Determine selectivity and strength of the resin.
- Physical properties: Durability, bead size, and porosity.
- Cation vs. anion: Matching the resin type to the problematic or target ions in the water.
By combining cation resins and anion resins appropriately, operators can design systems that address specific contaminants while maintaining water chemistry balance.
Regeneration of Ion Exchange Resins
Over time, the resin beads in an ion exchange system become saturated with the ions they’ve captured from the water. To restore their functionality, the resins must undergo a regeneration process, which displaces the accumulated ions and recharges the resin with its original exchangeable ions.
Why Regeneration Is Necessary
During operation, the resin’s exchange sites fill up with unwanted problematic ions such as calcium, magnesium, or chloride ions. Once the resin reaches its ion exchange capacity, it can no longer remove contaminants effectively, and regeneration is required.
How Regeneration Works
Regeneration involves passing a concentrated acid solution or brine solution through the resin bed. These chemicals replace the captured ions with the resin’s original ions.
For cation resins: Chemicals like hydrochloric acid, sulfuric acid, or sodium chloride are used to restore the resin to its hydrogen or sodium form.
For anion resins: Chemicals such as sodium hydroxide regenerate the resin by replacing the unwanted anions with hydroxide.
Key Chemicals Used
- Hydrochloric acid: Strong acid commonly used for cation resin regeneration.
- Sulfuric acid: Alternative to hydrochloric acid, depending on application.
- Sodium chloride: A safer, more neutral option for regenerating water softening resins.
- Sodium hydroxide: Required for regenerating anion resins.
After regeneration, the resin is rinsed and ready to resume removing scale forming ions, hardness ions, and dissolved salts. This process helps maintain resin efficiency, extends its lifespan, and ensures consistent water quality.
Read more on our full guide: What Is Ion Exchange Resin and How It Works
EAI’s Ion Exchange Technology Solutions
At EAI, we understand that maximizing water efficiency and minimizing operating costs starts with the right ion exchange technology. Our team specializes in designing and delivering site-specific ion exchange systems that address your unique water challenges — sustainably and effectively.
How EAI Facilitates Ion Exchange Reactions
Our experts analyze your facility’s makeup water, existing treatment program, and specific contaminants to design an optimal system. We use tailored combinations of cation resins and anion resins, carefully selected based on water chemistry and system needs.
We also ensure that the resin beds are properly engineered to support consistent ion exchange reactions, maintaining reliable removal of scale forming ions, dissolved salts, and other problematic ions.
What We Offer
- Customized resin bed configurations that balance functional groups and ion exchange capacity to meet your goals.
- Environmentally friendly approaches that reduce chemical consumption and water waste.
- Regeneration plans that minimize downtime and extend resin life.
- Solutions that support LEED credits and comply with manufacturer water quality standards.
Our resin-based systems enable higher cycles of concentration, reduced blowdown, and safer operation of critical equipment — with an attractive ROI.
Learn more about our Ion Exchange Resins for Makeup Waterservices.
Ready to Improve Your Water Efficiency?
We’re here to help you explore how tailored ion exchange technology can transform your water treatment process.
Let us know if resins can help your facility and discover the difference today.
Frequently Asked Questions (FAQs)
1. What is a weak acid cation (WAC) resin, and where is it used?
A weak acid cation (WAC) resin is a type of cation exchanger that contains carboxylic acid functional groups instead of sulfonic acid. WAC resins are ideal for removing alkalinity and specific metals in water with higher pH, and they operate differently from strong acid cation resins by targeting less problematic ions and providing efficient exchange under certain conditions.
2. How do anion exchangers and cation exchangers differ?
Anion exchangers are designed to remove negative ions like chloride, sulfate, and nitrate by exchanging them for hydroxide ions. Cation exchangers, on the other hand, target positively charged cations such as calcium, magnesium, and sodium, replacing them with hydrogen or sodium. Together, these systems enable the complete removal of other ions that contribute to water hardness, scaling, or corrosion.
3. What is the cation exchange process, and what role do exchangeable ions play?
The cation exchange process involves water flowing through a resin bed where exchangeable ions on the resin are swapped with hardness ions in the water. For example, calcium and magnesium are exchanged for sodium or hydrogen at the ion exchange sites. This removes scale forming ions and softens the water.
4. Why are physical properties and resin structure important?
The physical properties of a resin (such as bead size, porosity, and uniformity) directly affect water flow and contact with the resin. A stable resin structure and strong polymer structure ensure durability and prevent breakdown, maintaining consistent performance even after many regeneration cycles.
5. What chemicals are used in regeneration, and what do they do?
Several chemicals support resin regeneration:
- Brine solution or acid solution: Used in softening and demineralization to reset the resin.
These chemicals help remove dissolved ions and restore the resin’s ion exchange capacity.
- Sulfuric acid or hydrochloric acid: Recharges cation resins by replacing captured cations with hydrogen.
- Sodium hydroxide: Regenerates anion resins by restoring hydroxide ions.
6. Can ion exchange systems remove specific problematic compounds like calcium sulfate or carbonic acid?
Yes, properly designed systems can target and remove calcium sulfate, which contributes to scaling, and carbonic acid, which affects pH and alkalinity. Selecting the right resins and system design ensures even other ions and hard-to-remove contaminants are addressed effectively.